Illegal Chemistry Vol.2 - New/Novel Psychoactive Substances (NPS)

Alexander Shulgin died in 2014, but his legacy lives on. Breaking Bad finished, and captivated viewers in a way most producers couldn't have imagined. In the period of 2009-2017, 803 new substances of abuse had been reported onto the European Monitoring Centre for Drugs and Drug Addiction (EMCDDA) Early Warning System. Indeed, if any chemist felt themselves lacking inspiration, a quick visit to the EMCDDA database is all they would need.
The same websites are still around: Erowid, Bluelight; with a few new ones: PsychonautWiki, Drugs-Forum, and others. Reports and filings have documented the new kids on the block.
According to the last World Drug Report 2018 (United Nations publication, Sales No. E.18.XI.9), by the end of 2017 synthetic cannabinoids and synthetic cathinones represented the largest class of NPS, with 251 and 148 compounds detected, respectively. Most of them being sold online labelled as "plant food", "research chemicals", and other supposedly "legal" purposes. Hence the term, "legal highs".
A whole new crop of celebrity chefs have emerged with them, with the most infamous being libertarian Israeli chemist Dr Zee, or the "Godfather of Legal Highs". Running a legitimate company out of Amsterdam, he lectures to adoring rich kids:
"Those who know Dr. Zee's real name are able to follow him online on LinkedIn. They know that he is 47, was born in Israel, received his PhD in applied mathematics, and has applied for five patents. He designed algorithms for a large U.S. software company, worked on the "Human Genome Project," and spent time with an Israeli pharmaceutical company. Former colleagues praise him as being "highly talented, interesting, efficient and inspiring."
For chemists, the most exciting article ever to appear in Chemistry World was the story of how he re-invented Mephedrone while researching pesticides. It's an absolutely extraordinary read.
Generation X and the older Millennials have started their turn of the clandestine chemistry wheel. And, goodness, we are prolific. It seems we have learned the lesson of the Sixties Psychonauts: get them out faster than they can legislate them.
The Commission on Narcotic Drugs introduced the term "NPS" at the international level in its resolution 55/1 of 16 March 2012:
"The term 'new psychoactive substances' had been legally defined earlier by the European Union as a new narcotic or psychotropic drug, in pure form or in a preparation, that is not scheduled under the Single Convention on Narcotic Drugs of 1961 or the Convention on Psychotropic Substances of 1971, but which may pose a public health threat comparable to that posed by substances listed in those conventions (Council of the European Union decision 2005/387/JHA).
However, a large bulk of these latest compounds can trace their contemporary history back to Israel, of all places. The most prevalent found on online stores in 2011 were given as:
Kratom 128 92 Natural 6–15
Salvia 110 72 Natural 6–12
Hallucinogenic mushrooms 72 44 Natural 10–14
MDAI (aminoindane) 61 45 Synthetic 100–110
Methoxetamine (arylcyclohexylamine) 58 14 Synthetic 145–195
6-APB (benzofuran) 49 35 Synthetic 230–260
4-MEC (cathinone) 32 11 Synthetic 120–200
MDPV (cathinone) 32 25 Synthetic 115–239
Cactus 30 17 Natural 20–40 (plant)
Methiopropamine (thiophene) 28 5 Synthetic 115–130
5-IAI (aminoindane) 27 25 Synthetic 95–120
Dimethocaine (benzoate) 27 22 Synthetic 85–150
Methylone (cathinone) 26 17 Synthetic 76–130
5-APB (benzofuran) 23 6 Synthetic 250–330
AM-2201 (cannabinoid) 22 1 Synthetic 180–210
JWH-018 (cannabinoid) 20 5 Natural 200–230
JWH-250 (cannabinoid) 19 4 Natural 110–195
AMT (tryptamine) 19 13 Synthetic 230–460
MDAT (aminotetralin) 18 22 Synthetic 110–130
Mephedrone (cathinone) 18 23 Synthetic 120–200
JWH-122 (cannabinoid) 17 4 Synthetic 50–55/200–240
Ayahuasca (active principle DMT) 17 10 Natural 15–30 (kit)
4-FA (phenethylamine) 17 2 Synthetic 120–200
3,4-DMMC (cathinone) 16 3 Synthetic 90–200
Hawaiian baby woodrose (active principle
lysergamides) 16 10 Natural 4–8 (10 seeds)
GBL (GHB precursor) 15 12 Synthetic 35–45 (1⁄2 litre)Let's get into the chemistry.
Synthetic Cannabinoids: Spice/K2
Examples: JWH-018 (1-pentyl-3-(1-naphthoyl)indole), JWH-073 (1-butyl-3-(1- naphthoyl)indole), JWH-250, AM-2202, CP 47,497, cannabicyclohexanol, 5F-ADB, FUB-AMB, 5F-MDMB-PICA
As strangely-named as they are, most cannabinoids bear little resemblance to the alkaloids found in Cannabis Sativa or Indica. They are simply agonists of the same CB receptors found in the human brain with a 50-year history. As Cannabis legislation spreads, the need to fill the void in the black market grows: the answer being... chemicals sprayed onto herbs.
There are five major categories: classical cannabinoids, non-classical cannabinoids, hybrid cannabinoids, aminoalkylindoles, and eicosanoids. The first synthetic analogue of THC, HU-210, was first synthesized in Israel in 1988 and 100x more powerful. There are also five sets of synthetic families: CP-xxx, WIN-xxx, JWH-xxx, UR-xxx, and PB-xx; the most well known of which is the JWH suite, other than the AMs by (Alexandros Makriyannis) and RMs by (Raphael Mechoulam). 150+ have been observed in the wild to date, mostly aminoalkylindoles.
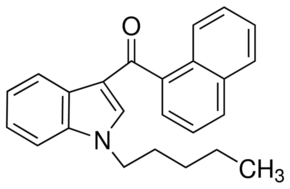
In the early 1990s, the John W. Huffman (JWH) research group at Clemson University synthesized over 450 cannabinoids. The most commonly found, and most notorious, is Naphthoylindole recipe 018 or 1-pentyl-3-(1-naphthoyl)indole, which we know as "Spice".
It's synthesis is well-circulated, and typically the simplest route is described thus:
"1H-indol-3-yl(naphthalen1-yl)methanone is prepared by Friedel-Crafts acylation using 1-H-indole and naphthalene-1-carbonyl chloride (prepared from naphthalene-1-carboxylic acid and thionyl chloride). Afterwards, N-alkylation is performed by addition of 1- bromopentane. The synthesis can also be performed vice versa. Common precursors are the above mentioned 1-H-indol, naphthalene-1-carbonyl chloride and 1-bromopentane. Alternatively, 1-pentyl-indole can be used as a precursor in order to skip the N-alkylation step."
Nobody needed them before. Legalise the plant, and a new market emerges.
- https://en.wikipedia.org/wiki/List_of_JWH_cannabinoids
- https://en.wikipedia.org/wiki/List_of_CP_cannabinoids
- https://en.wikipedia.org/wiki/List_of_AM_cannabinoids
- https://en.wikipedia.org/wiki/List_of_HU_cannabinoids
- https://en.wikipedia.org/wiki/Synthetic_cannabinoids
- https://en.wikipedia.org/wiki/List_of_miscellaneous_designer_cannabinoids
- http://www.emcdda.europa.eu/media-library/interactive-demystifying-chemistry-synthetic-cannabinoids_en
Synthetic Cathinones: Meow Meow/Bath Salts
Examples: mephedrone (4-methylmethcathinone, MEPH), methylone (3,4-methylenedioxy-N-methylcathinone), alpha-PVP/flakka (α- Pyrrolidinopentiophenone), 3,4-methylenedioxypyrovalerone (MDPV)
Cathinones are derived from the African shrub Catha edulis, commonly chewed as a stimulant named "Khat". They have been the next obvious development group for a long time in true analogue style. From Ephedra sinica we derive Ephedrine, which we can reduce/aminate to methamphetamine; if we oxidise it, we obtain ephedrone, most commonly known as methcathinone.
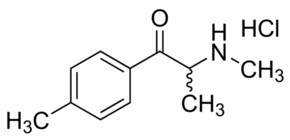
The theory behind synthetic cathinones - on a superficial read, of course - appears to be the Shulgin "essential oil" methodology: take the starting material, and apply substitutions as with MDA/MDE/MDMA etc.
The five most commonly seized cathinones in 2016 were alpha-PVP, 4-chloromethcathinone, 3-chloromethcathinone, 4-methyl-N,N-dimethylcathinone and 3-methylmethcathinone. The top five cathinones detected in powders were 4 chloromethcathinone (890 kg), 4-chloroethcathinone (247 kg), N-ethylhexedrone (186 kg), 3-methylmethcathinone (126 kg) and mexedrone (50 kg).
The most infamous, of course, is mephedrone (4-methyl methcathinone, 1-(4-methylphenyl)-2-methylaminopropanone) which was first created in 1929 as toluyl-alpha-monomethylaminoethylcetone and "rediscovered" in 2003 by Hive user Dr Zee ("kinetic"). It was the first mainstream online legal high to be mass-produced in Israel and marketed as "plant food" for general consumption. The story itself is worthy of a Hollywood film:
"‘Its production method was reverse engineered by Chinese labs,’ Zee recalls. ‘Then it spread all over Europe and people said “What have you done?” – and I said, “Oh my god, what have I done?”’
Next, obviously, was the 4-fluoro variant (flephedrone), MDMA-style (methylone, created by Shulgin in 1996), and pyrovalerone (MDPV) types.
Synthesis is simple via 2 different routes: a) oxidation of 4-methylephedrine with potassium permanganate (the "bathtub" version), or b) the methylation of 4'-methyl-2-bromopropiophenone with methylamine and triethylamine dissolved in dichloromethane.
Take the cathinone molecule base, add the same PIHKaL substitutions. Repeat.
As the EMCDDA notes:
"The ring-substituted N-methylcathinone derivatives are best synthesised by reacting the suitably substituted bromopropiophenone with methylamine; the result is always racemic. In the case of methylone, for example, 2-bromo-3,4-methylenedioxy-propiophenone can be prepared by reacting 3,4-methylenedioxypropiophenone with bromine. These precursor substances are readily available and none of them is under international control."
- http://www.emcdda.europa.eu/publications/drug-profiles/synthetic-cathinones
- https://en.wikipedia.org/wiki/Substituted_cathinone
Synthetic Piperazines: Rapture
Examples: Benzylpiperazine (BZP), para-Fluorophenylpiperazine (pFPP), para-Methoxyphenylpiperazine (MeOPP), 1-methyl-4-benzylpiperazine (MBZP), meta-Chlorophenylpiperazine (mCPP), dibenzylpiperazine (DBZP), 3-Trifluoromethylphenylpiperazine (TFMPP)
Piperazines are a class of drug which have a real whiff of "cheap yuck" about them. Originally designed for use in agriculture, they have been historically added to street amphetamines as the effects are comparable, albeit at ~10% of the potency. They were originally named because of their chemical similarity with piperidine, part of the structure of piperine in the black pepper plant (Piper nigrum).
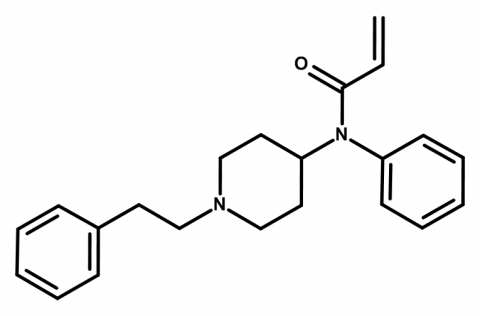
Many of the piperazines are so unpleasant they could easily be used for aversion therapy: headaches, insomnia, and psychosis.
Synthesis of BZP is trivial: piperazine monohydrochloride + benzyl chloride. As for mCPP, the EMCDDA notes it can be accomplished in two main ways:
"the reaction of diethanolamine with m-chloroaniline. Other methods involve the reaction of m-chloroaniline with bis(2-chloroethyl)amine or the reaction of piperazine with m-dichlorobenzene."
- http://www.emcdda.europa.eu/publications/drug-profiles/bzp
- https://en.wikipedia.org/wiki/Substituted_piperazine
- https://en.wikipedia.org/wiki/Phenylpiperazine
Substituted Phenethylamines: 2Cx/NBOMe Families
Examples: 2C series (2C-E, 2C-B, 2C-I), 4-FMA, NBOMe series (25I, 25C, 25B), PMMA, 5-APB, 6-APB, D series (DOI, DOC), Ethylbenzodioxolylbutanamine (EBDB; Ethyl-J), Methylbenzodioxolylpentanamine (MBDP; Methyl-K), (Bromo-Dragonfly)
With the most infamous - read: desirable - of Shulgin's PIKHaL catalogue already added to the naughty list and their precursors firmly locked down, it's become time to explore the less interesting remaining compounds and their modifications. With 2-CB firmly established as the most prominent essential oil substitution, Bromo-DragonFly is not far behind. During 2010, nearly two-fifths of 2C-phenethylamines were identified as 2C-B (39%), 33% as 2C-E, and 23% as 2C-I.
Many of the newer compounds emerging on the black market are derived from the work of David E. Nichols in the 1990s: his children include new LSD derivatives (ETH-LAD, PRO-LAD, and AL-LAD), 4-methylthioamphetamine (MTA), 5-methyl-MDA, 4-MTA, MDAI, 2CBCB-NBOMe, and TCB-2.
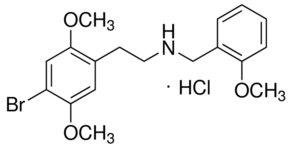
Shulgin's 2C-B (2,5-dimethoxy-4-bromophenethylamine) in itself is relatively simple to understand as are most of the 2-C family. 2C-E uses an ethyl group, whereas 2C-I (4-iodo-2,5-dimethoxyphenethylamine) imports the iodo configuration. The DOx series which follows them are slight modifications: 2,5-Dimethoxy-4-chloroamphetamine (DOC), 2,5-Dimethoxy-4-iodoamphetamine (DOI), etc.
Nichol's team's research naturally led them down the Benzodifurans path, producing the two most notable entries: 25I-NBOMe (25L, from the 25-NB family) and Bromo-DragonFLY (1-(8-bromobenzo[1,2-b;4,5-b']difuran-4-yl) -2-aminopropane), one of the 3 "FLY" family.
The former is an analogue of 2C-I, formed by adding a 2-methoxybenzyl (BnOMe) onto the nitrogen (N) of the phenethylamine backbone via reductive alkylation with 2-methoxybenzaldehyde. The latter's synthesis is almost comical in how widely circulated it is despite the original paper appearing so banal, yet being far more complicated:
"Benzodifuran 3 was prepared as depicted in Scheme 1. The aminopropane 4, available from our previous synthetic work, 5 was protected as the trifluoroacetamide 5. Bromination with Br2 gave 6, which was then oxidized with dichlorodicyanobenzoquinone (DDQ) in toluene, yielding the aromatized derivative 7. Deprotection produced the free amine that was neutralized with ethanolic HCl and precipitated from ether as fine white crystals of the hydrochloride salt of 3."
It would be comical, if it weren't for all the deaths associated with 4-MTA and DragonFLY.
- https://en.wikipedia.org/wiki/Substituted_phenethylamine
- https://en.wikipedia.org/wiki/Substituted_amphetamine
- https://en.wikipedia.org/wiki/Substituted_methylenedioxyphenethylamine
- https://en.wikipedia.org/wiki/Substituted_benzofuran
Novel Stimulants: Amino-indanes/tetralins, Oxazolines
e.g. 5,6-methylenedioxy-2-aminoindane (MDAI), 5, 6-methylenedioxy-N-methyl-2-aminoindane (MDMAI), 5-iodo-2-aminoindane (5-IAI), and 5-methoxy-6-methyl-2-aminoindane (MMAI), 6,7-Methylenedioxy-2-aminotetralin (MDAT), 4,4'-Dimethylaminorex
Although they follow a similar pattern to the phenethylamine substitution patterns, aminoindanes have a substantially different "backbone" structure granting them different properties: an indane group has a benzene ring fused to an additional cyclopentane ring: the cyclopentane can almost be thought of as replacing the amphetamine-style branch. A tetralin group is a bicyclic/duo set of benzene rings fused together. So the former has 5 points (or 11, together), and the latter has 6 (or 12, together).
Oxazolines have been well-known for many years as appetite-suppressants, anorectic, or "diet pills". Unsurprisingly, stimulants tend to have these properties, and anorectics tend to have stimulant properties. The favourites include Aminorex (5-phenyl-4,5-dihydro-1,3-oxazol-2-amine), and 4-methyl Aminorex (2-Amino-4-methyl-5-phenyl-2-oxazoline).
The most infamous so far were developed David Nichols' team alongside the newer phenethylamines.
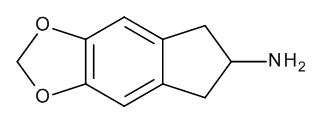
Most of Nichol's indane and tetralin amphetamine analogues follow the same Shulgin methodology: build the backbone of the proposed molecule with an amine group, then apply substitutions in the familiar patterns. MDAI uses a 5,6-methylenedioxy block; MDMAI compounds that with a n-methyl addition. MEAI uses a 5-methoxy group; MMAI a 5-methoxy n-methyl modification, and 5-IAI an iodo block. The Aminotetralins build on the 2-Aminotetralin pattern, with 6-Chloro, 6,7-Methylenedioxy, and 6,7-methylenedioxy-N-methyl (MDMAT) substitutions.
The syntheses of each group are relatively simple and many documented in Nichols' journal papers: for MDAI, from 3-(3,4-methylenedioxyphenyl)propionic acid to 5,6-Methylenedioxy-1-indanone, finally to a reduction to the 2-aminoindan. Tetralins are generally reduced from their tetralone parent. 4,4 DMAR follows a similar route as the other Aminorex compounds: transformation of a 4-methylnorephedrine precursor using cyanogen bromide or potassium cyanate (!).
- https://en.wikipedia.org/wiki/List_of_aminorex_analogues
- https://www.dea.gov/sites/default/files/pr/microgram-journals/2011/mj8-2_43-52.pdf
- https://en.wikipedia.org/wiki/Category:Tetralins
Substituted Tryptamines
Examples: DMT, 5-Meo-DMT, 5-Meo-DPT, AMT, 4-AcO-DMT (O-Acetylpsilocin), 4-Acetoxy-DiPT
Chatter around DMT has exploded almost in line with the growth of the Internet itself. Ayahuasca "retreats" are almost mainstream, as podcasters openly tout the drug as a form of homeopathy. The seminal reference for the largest reservoir (55) of tryptamine derivatives is, of course, TIKHal, by Papa Shulgin. Their allure, as stands to reason, is their appearance in nature - i.e., their "natural" status.
Tryptamines (indolealkylamines) are indeed fascinating substances. They include serotonin, tryptophan, psilocybin, melatonin, and bufotenin. The question for philosophers is why they are so prevalent in nature; the question for chemist hackers is how they can be modified to increase their effects.
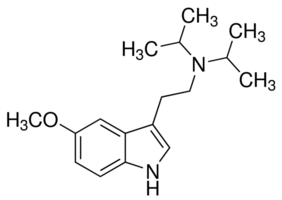
DMT doesn't require synthesis as it could even be extracted by a child from Acacia confusa or Mimosa hostilis. 5-Methoxy DMT has many more. What makes the tryptamines so interesting for clandestine chemists is the wide availability of over-the-counter precursor chemicals like 5-Methoxytryptamine (Mexamine), and the numerous plants which contain starting blocks.
The production of alpha-MethylTryptamine (AMT) has well known routes ("Nitroaldol Condensation between indole-3-carboxaldehyde and nitroethane under ammonium acetate catalysis and the condensation between indole-3-acetone and hydroxylamine followed by reduction of the obtained ketoxime with lithium aluminum hydride").
Shulgin details 5-MeO-DPIT's synthesis in glorious simplicity: from melatonin, to 5-methoxytryptamine, finally reacted with diisopropylethyl amine and 2-iodopropane. The subjective reports are colourful, to say the least.
- https://en.wikipedia.org/wiki/Substituted_tryptamine
- https://en.wikipedia.org/wiki/List_of_naturally_occurring_tryptamines
Fentanyls / Synthetic Opioids
Examples: AH-7921; MT-45, butyrylfentanyl (BF), p-Floro-BF (PFBF), 4F-Butyr Fentanyl (4F-BF), U-47700
Why anyone would want to mess around with these compounds, whose street versions could easily be categorised as weapons of mass destruction, is anyone's guess. Fentanyls (e.g. tranquilizer dart Carfentanil, the nightmare scenario) aren't funny; they are deadly not only because of their toxicity, but because they suppress breathing. Canadians, particularly, are partial to these compounds. If there's one group of chemicals - other than methamphetamine and its immediate derivatives - which tend to horrify chemists and locate their ethical streak, it's these.
China, in one word. at least 12 different fentanyls have been found in the US: Acetyl-
alphamethyl, Alfentanil, Alpha-methyl, Alpha-methylthio, Beta-hydroxy, Beta-hydroxy-3 methyl, 3-methyl, 3-methylthio, Para-fluoro, Remifentanil, Sufentanil, Thio, and Carfentanil. Ironically, the Butyr series are less potent, which almost certainly has to be a business decision.
The newer, simpler synthesis of Fentanyl (N-(1-(2-phenethyl)-4-piperidinyl-N-phenyl-propanamide) is via the Siegfried method: the creation of precursor N-phenethyl-4-piperidone (NPP) by reacting 4-piperidone hydrochloride with phenethyl bromide. Butyrfentanyl's synthesis is protected by a 1965 patent which is freely available.
The fentanyls have such broad medical use they are well-studied and the information detailing their industrial manufacture is documented throughout academic literature.
There is no getting around what these compounds are and what they do. If you make them and distribute chemicals designed to take out rhinos and elephants, to ordinary humans, you know full well what happens next.
- https://en.wikipedia.org/wiki/List_of_fentanyl_analogues
- http://www.emcdda.europa.eu/publications/drug-profiles/fentanyl
Dissociative Anaesthetics
Examples: 3-MeO-PCE, 4-MeO-PCP, Methoxetamine (MXE), Eticyclidine
With Ketamine and Phencyclidine firmly out of vogue as medical essentials and/or potential anti-depressants compared to their status as illegal psychoactives, the next stage has been to produce modified compounds which duplicate their effects. Coincidentally, it was Alexander Shulgin who supplied the main data on illicit PCP production around 1976.
The predominant PCP methoxy arylcyclohexylamines are 3-Methoxy Eticyclidine (3-methoxy PCE) and 3/4-methoxy PCP, all of which are well-described in chemical literature and easily available for study (e.g. "Syntheses and analytical characterizations of N-alkyl-arylcyclohexylamines"). The former from 3/[1/(3/methoxyphenyl)cyclohexyl]acetamide, and the latter via the Grignard of Piperidine cyclohexane carbonitrile (PCC).
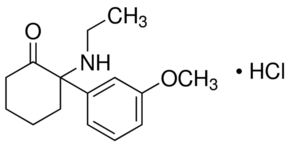
Methoxetamine (2-(3-Methoxyphenyl)-2-(ethylamino)cyclohexanone) first appeared on the black market and has been touted as the "next, better Ketamine" for some time, and again, routes to its synthesis are now well-known:
"A cyclopentyl Grignard was reacted with 3-methoxybenzonitrile to form 3-methoxyphenyl cyclopentyl ketone, which was then brominated alpha to the ketone. The alpha-bromo ketone was converted to the Schiff’s base with ethyl amine, which was then heated to form methoxetamine."
Kratom
Kratom is a pseudo-homeopathic herb sold online a pain-relieving stimulant and opiate withdrawal-suppressor, and recently escaped wide-scale bans despite being on the DEA's "drugs of concern" list. It is derived from the oval-shaped white-green leaves of an evergreen tree in the coffee family, Mitragyna speciosa, which is found in South East Asia (mainly Thailand, but also Indonesia, Malaysia, Myanmar, and Papua New Guinea).
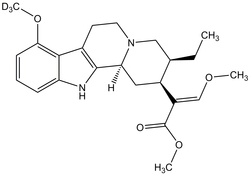
The two most prominent alkaloids amongst the 40 found in the plant are indole-based opioid-receptor agonists mitragynine (66%) and more potent 7-hydroxymitragynine (7-HMG, 2%). They are named for the way parts of the plant resemble the shape of a bishop’s mitre.
There is no reasonable argument for the artificial production of Kratom alkaloids in a laboratory setting.
Synthetic Tropanes (Cocaine Analogues)
Examples: 4-fluorococaine, 2-Hydroxycocaine, 3-(p-fluorobenzoyloxy)tropane, dimethocaine.
There are three main problems with producing cocaine substitutes in a lab: a) Erythroxylum coca is a more cost-effective natural lab, b) the dental side-effects are off-putting, and c) the potency tends to kill your customers. However, when the risks of agriculture outweigh those of the lab, it's inevitable new series' will emerge.
The bulk of work pertaining to benzoic acid esters was done in 1973 by Robert Clarke. There are 200+ known analogues in journal literature with differing properties, of which 4-Fluorococaine is arguably the best known from its inclusion in Future Synthetic Drugs of Abuse, but despite its supposed 60x strength is reported to be a miserable experience.
The most contemporary group of analogues are the RTI series, of which two (3β-(4-chlorophenyl)- (RTI-COC-31) and 3β-(4-methylphenyl)-tropane-2-carboxylic acid methyl ester (RTI-COC-32), or RTI-31 and RTI-32), are believed to also have 50-100x potency, yet require cocaine as the starting material.
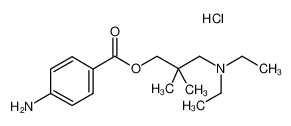
Despite doubts about its psychoactivity, dimethocaine/larocaine (best described as an analogue of procaine, the dental anaesthetic) is appearing more frequently in online stores. Methods of its synthesis (the p-aminobenzoyl ester of 2,2-dimethyl-3-diethylamino-1-propanol) are well-known and cite the condensing of 4-aminobenzoic acid ethyl ester with diethylamino-t-butanol.
- https://en.wikipedia.org/wiki/List_of_cocaine_analogues
- http://www.emcdda.europa.eu/publications/drug-profiles/synthetic-cocaine-derivatives
Non-Medical Benzodiazepines
Examples: Pyrazolam, diclazepam, flubromazepam, etizolam
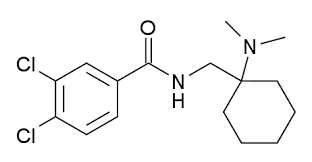
Benzos aren't exactly sexy chemicals. Addicts steal them from pharmacies, and their medical use since the 50s is without question. Theft is simpler and cheaper than laboratory production. In fact, one of the largest black market usages of this family of compounds is the countering of the usage of another.
The sale of benzos is notable for the simple reason they are marketed as a form of light "party opiate" or "fake morphine". An example being "Pinky", or 3,4-dichloro-N-[2-(dimethylamino)cyclohexyl]-N-methylbenzamide. Entire lists have been newly-added to classification lists.
- http://www.emcdda.europa.eu/publications/drug-profiles/benzodiazepine
- https://en.wikipedia.org/wiki/List_of_benzodiazepines
The People Behind The Chemistry
One man's meat is another's poison; one man's antidepressant is another's psychedelic. The molecules are one thing, but their authors are equally as intriguing. Although the days of the cult chemists, like Uncle Fester, Strike, Rhodium, or Alexander Shulgin, are all but gone, the next generation have already sprung up like flowers; albeit with many of them having inadvertently contributed by proxy through their journal articles and/or well-meaning work within other areas.
To study the work behind these chemicals, it's worth reviewing the journal submissions and media featurettes of the following:
- Robert Clarke et al (synthetic cocaine, 1970s)
- John W. Huffman et al (synthetic cannabinoids, 1990s)
- Alexandros Makriyannis et al (synthetic cannabinoids, 1990s)
- Raphael Mechoulam at al (synthetic cannabinoids, 1990s)
- David E. Nichols et al (psychedelic amphetamines, 1990s)
- Dr Zee (synthetic cathinones, 2000s)
- M (Ketamine derivatives, 2010s)
Further Reading
For more information, the canonical reference for NPS data is the masterful "Novel Psychoactive Substances: Classification, Pharmacology and Toxicology" by Paul Dargan and David Wood.
For prevalence, the DEA's National Forensic Laboratory Information System Annual Report is the master source of market activity.