A Novel Method For Producing MDMA From Moskachan A

Theoretical chemistry is tremendous fun. It can be a wonderful intellectual challenge. MDMA is a fantastic ChemGolf target because, like most phenethylamines, the production routes are well-known and internationally-controlled at global and local level. Can we theoretically find a way to produce MDMA which gets around the precursor blacklists as an educational and intellectual exercise?
Yes. Meet Moskachan A. Aka 3',4'-(Methylenedioxy)acetophenone.
Note: we won't be going into a "recipe" here, but organic chemists will understand the rationale and methodology behind the steps.
How Is MDMA Already Produced?
MDMA has a unique 3,4-methylenedioxy group which gives it a key signature. It is a substituted amphetamine. It's chemical name is 3,4-Methylenedioxymethamphetamine.
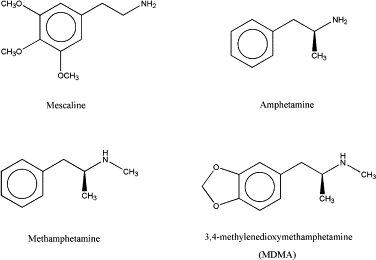
The main chemicals in nature with this "backbone" structure are Safrole (5-(Prop-2-en-1-yl)-2H-1,3-benzodioxole), Isosafrole (isomer), Myristicin (3-methoxy-4,5-methylenedioxy-allylbenzene), and Piperonal (heliotropin, 2H-1,3-Benzodioxole-5-carbaldehyde).
Almost all the methods do the same thing: convert the natural "backbone" to an intermediate ketone (the phenyl-2-propanone derivative, aka MDP2P), and reduce it with a basic amine to produce the final amphetamine. The synthesis is trivial. It's boring.
- Safrole -> 2-bromopropane -> (methylamine) -> MDMA
- Safrole -> phenyl-2-propanone (MDP2P) -> (methylamine) -> MDMA
- etc etc
How do we stop that?
- Ban the cultivation of plants;
- Monitor the traffic of methylamine (watch "Breaking Bad").
Safrole is heavily controlled via the INCB: https://www.incb.org/incb/en/precursors/Red_Forms/red-list.html
The search for it has created staggering destruction in natural South East Asian forests, particularly in Northern Cambodia.

"Sassafras oil processing plants are usually located besides streams to provide water for boiling and cooling the distilled oil," Bradfield said. The oil leaks into the streams, wreaking yet more environmental damage. "There are frequently dead fish and frogs floating in the streams near these distilleries," Bradfield said. The water from this area flows down into the rest of Cambodia through the Mekong and Tonle Sap rivers."
https://www.thenewhumanitarian.org/report/79340/cambodia-ecstasy-tabs-destroying-forest-wilderness
More: https://www.unodc.org/unodc/en/frontpage/cambodia-tackles-safrole-oil-production.html
The Ruta Genus
The Ruta genus, commonly known as rue, is a group of evergreen shrubs native to the Mediterranean region, Macaronesia, and southwest Asia. These weeds are known for their strong, often unpleasant odour and bitter taste. Ruta species have pinnately divided leaves and yellow flowers with 4-5 petals, typically blooming in summer. Many have been used in traditional medicine and culinary applications, although their use has declined due to potential toxicity and photosensitising effects.
These things smell and taste disgusting. Because they're bloody poisonous.
Ruta angustifolia, also known as narrow-leaved rue, is a species native to the western Mediterranean region, including Spain, Portugal, France, and Italy. It is a small, evergreen shrub growing up to 50 cm tall, with narrow, linear leaflets and small, yellow flowers. This species prefers well-drained, rocky soils and full sun exposure.

Ruta chalepensis, commonly known as fringed rue or Aleppo rue, is a species native to the Mediterranean region, extending from the Canary Islands and Madeira to the Arabian Peninsula and East Africa. It is a small, evergreen shrub growing up to 80 cm tall, with compound leaves consisting of oval to oblong leaflets with fringed margins. The flowers are yellow and appear in summer. Ruta chalepensis is adapted to dry, rocky habitats and is often found in disturbed areas, such as roadsides and abandoned fields. This species has been introduced to various parts of the world, including the Americas and Australia, where it can become naturalized or invasive.

Both are perennial plants with a woody base. They can be propagated by seeds or cuttings and are relatively drought-tolerant once established.
The Moskachan Alkaloids
According a 1991 article in the Journal of Essential Oil Research:
Forty-one compounds have been identified in the essential oil of Ruta angustifolia. Besides the already known compounds, seven derivatives possessing the 3,4-methylenedioxyphenyl moiety were identified, including piperonyl acetone and a novel olefinic derivative 8-(3,4-methylenedioxyphenyl)-1-octene.
https://sci-hub.ru/10.1080/10412905.1991.9697956
Joulain, D., Laurent, R., Fourniol, J. P., & Yaacob, K. B. (1991). Novel moskachan related compounds in the essential oil of Ruta angustifolia Pers. from Malaysia. Journal of Essential Oil Research, 3(5), 355-357.
According to Bailly (2023):
A relatively rare series is called moskachans A-D, four benzodioxone derivatives, discovered initially from the aerial part of R. angustifolia and later found in R. chalepensis. The name moskachan comes from ‘moskatxa’, the Basque name for R. angustifolia but also used for other Ruta species. Moskachan B is not cytotoxic whereas moskachan D displays a weak antiproliferative activity against A549 lung cancer cells (IC50 = 74.4 μM).
In addition to these four moskachans, other compounds with a methylenedioxyphenyl moiety have been identified from an essential oil of R. angustifolia (from a specimen collected in Malaysia), including compounds structurally close to safrol which is a well-known phenylpropene derivative. Moskachans have been occasionally found in other species but rarely studied. They could be further considered as ingredients in the food or perfumery industry, as it is the case for other compounds with a methylenedioxyphenyl moiety.
Bailly, C. (2023). Ruta Angustifolia Pers.(narrow-Leaved Fringed Rue): pharmacological properties and phytochemical profile. Plants, 12(4), 827: https://www.mdpi.com/2223-7747/12/4/827
The compounds of interest are:
- 4-(3,4-methylenedioxyphenyl)-2-butanone (0.05%)
- 4-(3,4-methylenedioxyphenyl)-2-butanol (0.02%)
- 4-(3,4-methylenedioxyphenyl)-2-butyl acetate (0.28%)
- 6-(3,4-methylenedioxyphenyl)- 2-hexanone (0.53%)
- 8-(3,4-methylenedioxyphenyl)- 1 -octene (2.13%)
- 8-(3,4-methylenedioxyphenyl)-2-octanone (2.98%)
The 4 "Moskachans" are quite interesting indeed:
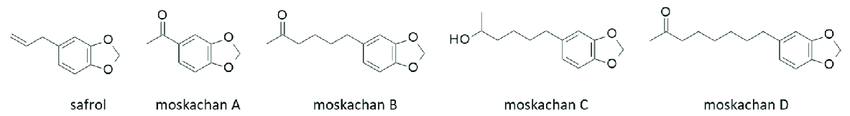
- Moskachan A: l-acetyl-3,4-methylenedioxybenzene
- Moskachan B: 6-(3,4-methylenedioxyphenyl)-2-hexanone (R= (CH2)4-CO-Me)
- Moskachan C: 6-(3,4-Methylenedioxyphenyl)-2-heptanol (R= (CH2)4-CHOH-CH3)
- Moskachan D: 8-(3,4-methylenedioxyphenyl)-2-octanone (R= (CH2)6-CO-Me)
Other references:
- Del Castillo, J. B., Secundino, M., & Luis, F. R. (1986). Four aromatic derivatives from Ruta angustifolia. Phytochemistry, 25(9), 2209-2210. https://sci-hub.ru/10.1016/0031-9422(86)80093-0
- Ulubelen, A., & Tan, N. (1990). A moskachan from roots of Ruta chalepensis. phytochemistry, 29(12), 3991-3992. https://sci-hub.ru/10.1016/0031-9422(90)85390-2
- Ulubelen, A., & Öztürk, M. (2006). Alkaloids and coumarins from Ruta species. Natural Product Communications, 1(10), 1934578X0600101006. https://sci-hub.ru/10.1177/1934578X0600101006
Moskachan A to MDMA
Compounds B - D are of no real use because the C-C sidechains are too difficult to deal with in a practical way.
Moskachan A (1-(3,4-Methylenedioxyphenyl)ethanone), however, is simple. It features a benzene ring substituted at the 1-position with an acetyl group (COCH3) and at the 3- and 4-positions with a methylenedioxy group (-O-CH2-O-).
Crystalline solid with a MP of 80-85°C, soluble in the usuals (ethanol, methanol, chloroform etc). Characteristic aromatic proton signals in the region of 6-8 ppm, acetyl methyl group signal around 2-3 ppm. Presence of carbonyl stretch around 1700 cm^-1, methylenedioxy group stretch around 1100-1150 cm^-1.
This is easy stuff. All we need to do is modify the acetyl/acetophenone group.
A one-pot process could start with the conversion of Moskachan A into an epoxide using m-chloroperbenzoic acid (m-CPBA). Subsequently, dimethylamine would be directly introduced to the reaction, opening the epoxide ring and yielding N,N-dimethyl-3,4-methylenedioxyphenyl-2-epoxypropanone.
1 . MDAcP + m-CPBA → 3',4'-(Methylenedioxy)phenyl-2,3-epoxypropanone (3,4 MDP23EP)
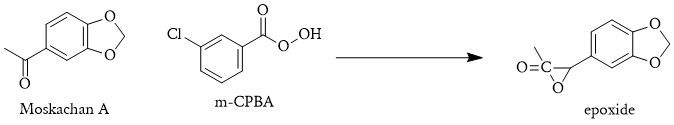
2. 3,4 MDP23EP + NH(CH3)2 → N,N-Dimethyl-3,4-methylenedioxyphenyl-2-hydroxypropanone (DM3,4MDP2HP)
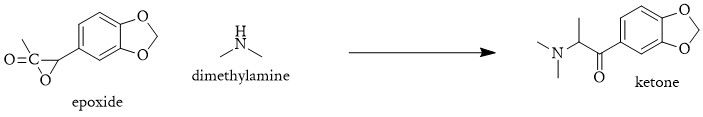
3. DM3,4MDP2HP + NaBH4 → MDMA
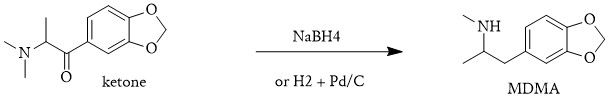
Finally, sodium borohydride (NaBH4) would reduce this intermediate to MDMA, completing the synthesis in a single reaction vessel. This kind of streamlined method minimises purification steps and simplifies the overall synthesis.
A one-pot would look like this:
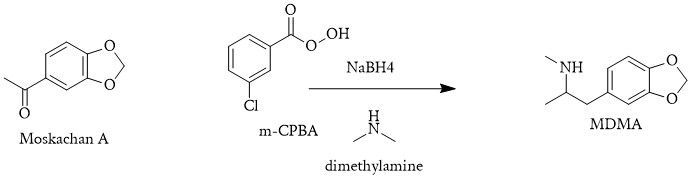
- The acetophenone can be converted into an epoxide using a peracid (e.g. m-CPBA). This introduces a strained three-membered ring, setting up the molecule for nucleophilic attack.
- The epoxide ring is opened using dimethylamine, leading to the formation of a β-hydroxy ketone with the dimethylamino group introduced at the β position (if using a one-pot, otherwise epoxide). This avoids the use of methylamine directly.
- The β-hydroxy ketone is reduced to a β-hydroxy amine, effectively forming MDMA. The final product is obtained by reducing the carbonyl to a secondary alcohol using a mild reducing agent like NaBH4 or a stronger reducing agent like LiAlH4.
- Purification can be achieved by standard techniques like recrystallisation or column chromatography.
To avoid using DMA (dimethylamine), ammonia and formaldehyde can be used directly to achieve a ring-opening via Mannich-type reaction and to introduce the N-methyl group into the intermediate.
To replace NaBH4, catalytic hydrogenation using H2 gas and palladium on carbon (Pd/C) could be employed to reduce the carbonyl to the desired alcohol.
Conclusion
This is a theoretical thought experiment and would never be applicable to real-world industrial production simply on the basis the % amounts are too small. Sassafras Oil is sought after because it is so densely rich in Safrole.
At 2.98%, Moskachan D is the most prevalent alkaloid. Adapting this one is trickier, but B-D could roughly be accomplished by:
- Converting the octanone (in the case of D) to the benzeneacetaldehyde derivative, using sodium periodate (NaIO4), osmium tetroxide (OsO4) or KMnO4, or alternative oxidative cleavage reagents;
- Reducing the benzeneacetaldehyde derivative to the corresponding alcohol with NaBH4 or LiAlH4.
- Converting the alcohol to the corresponding bromoalkane using phosphorus tribromide (PBr3) or thionyl chloride (SOCl2) with HBr;
- Substituting the bromine with a methylamine group to form MDA (using methylamine);
- Methylating the amine group to form MDMA with formaldehyde, and sodium cyanoborohydride (NaBH3CN);
Hopefully this goes some way to illustrating the purposelessness of current synthetic drug policy under Pax Americana. You simply can't stop knowledge and ideas, and there's always a way around things if you bother to sit down and work it out. You can legislate it away.
